Abstract

Introduction: Post-transplant cyclophosphamide (PTCy) is a commonly used prophylactic therapy for graft-vs-host disease (GVHD), particularly in the setting of haploidentical (haplo) transplantation. Despite prior studies showing comparable survival outcomes when compared to HLA matched unrelated donor transplantation (MUD-HCT) using traditional calcineurin inhibitor based GVHD prophylaxis, the rate of graft failure has been reported to be as high as 12-20% in haplo-HCT recipients. In the current study, we report the impact of PTCy on engraftment failure and overall mortality in AML, ALL and MDS patients after haplo vs MUD HCT receiving a uniform PTCy GVHD prophylaxis to determine if excess late graft failure is due to PTCy, donor source or a combination of both factors.
Methods: We performed a retrospective cohort analysis using the Center for International Blood and Marrow Transplant Research database among adult patients who underwent first reduced intensity conditioning (RIC) haplo or 8/8 MUD allogeneic HCT between 2011 and 2018 for AML, ALL or MDS with PTCy GVHD prophylaxis previously described by Gooptu et al (Blood 2021). The two comparative cohorts were: 1.) haplo recipients receiving GVHD prophylaxis with PTCy, calcineurin inhibitor/mTOR inhibitor and mycophenolate, and 2.) MUD-HCT recipients receiving GVHD prophylaxis with PTCy, calcineurin inhibitor/mTOR inhibitor and mycophenolate. The population was restricted to patients that initially engrafted after transplant. The primary outcome was incidence of late graft failure, defined as any graft failure following primary neutrophil engraftment. We sought to identify risk factors for graft failure in the two cohorts. Secondary outcomes were overall mortality and its associated risk factors in the haplo vs. MUD-HCT cohorts.
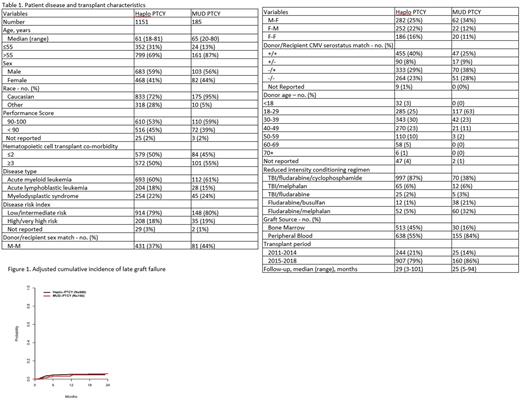
Results: A total of 1336 patients met the eligibility criteria (N=60 haplo and N=2 MUD excluded due to a lack of initial engraftment). Of these, 1151 patients received a haplo-HCT and 185 received a MUD-HCT. Patients in the Haplo group were older (61 vs 65), more ethnically diverse (28% vs 5% non-Caucasian), received more bone marrow grafts (45% vs 16%), had older donors (median age 37 vs 28 years old), received predominately TBI/Cy/Flu regimens (86% vs. 32%) and had longer median follow-up (29 vs 25 months) (Table 1). At 2 years, the adjusted probabilities of late graft failure for the haplo-HCT group was 6.5% (95% CI 5.2-8.0) vs 5.9% (95% CI 2.7-10.9) for the MUD (p = 0.785). The 2-year overall survival, however, was significantly lower for the haplo-HCT cohort, 56% (95% CI 53-39) vs 69% (95% CI 61-75) for the MUD-HCT group (p=0.001). Multivariate analysis to factors associated with increased risk of graft failure demonstrated that a diagnosis of MDS, HR 1.98 (95% CI 1.22-3.20, p=0.005), and being transplanted in the earlier time period (2015-2018 vs 2011-2014 HR 0.39, 95% CI 0.24-0.64, p =0.0002). Multivariate analysis to evaluate risk factors associated with overall mortality demonstrated that haplo-HCT, HR 1.46 (95% CI 1.11-1.91, p-value = 0.007), high risk disease, HR 2.31 (95% CI 1.87-2.84, p=<0.0001), HCT comorbidity score of >/= 3, HR 1.34 (95%CI 1.13-1.58, p=0.0007), and a diagnosis of ALL, HR 0.51 (95%CI 0.39-0.67, p=<0.001) were all significantly associated with increased risk. A post-hoc sensitivity analysis to evaluate the effect donor age between donor types on graft failure was performed. Cases and controls were selected where donor age was between 18-49 years old, resulting in a sub-cohort of 1078 patients (898 haplo and 180 MUD). When adjusted for donor age, graft failure did not differ between haplo and MUD HCT HR 1.19(95% CI 0.53 - 2.69, p=0.6731) (Figure 1).
Conclusion: In this registry-based analysis of patients undergoing reduced intensity transplant for AML, ALL or MDS using GVHD prophylaxis with PTCy, there was no significant difference in late graft failure rates when utilizing a haplo vs. MUD-HCT with PTCy for GVHD prophylaxis. Overall mortality, however, was significantly higher in the haploidentical donor cohort, as previously reported.
Disclosures
Zhang:Astellas Pharma Inc: Research Funding; Bristol Myers Squibb: Research Funding; Gamida Cell: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal